

This fluid type can be used to model fluids without phase transition, whose properties are not dependent on the pressure. Typical applications are thermal oils and molten salt.
These fluids are available:
The thermal oils Helisol 5A, Helisol XA and Helisol XLP from Wacker have also been included in the standard database, each in an unused and a used (i. e. after 750 operating hours) variant and each for the four pressure levels 1 bar, 10 bar, 20 bar and 30 bar.
In contrast to the other integrated thermal oils, the Helisol data is not interpolated with polynomials, but with characteristic curves. In the calculation, only the dependence on the temperature is considered, but not the dependence on the pressure. This can only be taken into account by selecting the most suitable data set. A plausibility check with regard to the line pressure does not take place.
Heat transfer oils from Fragol: As it was stated by the manufacturer that Fragoltherm DPO can also be used in the gas phase, both ‘Fragoltherm DPO liquid’ and ‘Fragoltherm DPO gaseous’ were included. However, it should be noted that no additional data on the pressure dependency was provided for the gas phase. Neglecting the pressure dependence is certainly accurate enough for modelling the heat transfer. However, the density is only correct in the vicinity of the saturated vapour point, and the approximation as an incompressible fluid is likely to lead to a significant underestimation of pump or compressor performance.
There are also some PCM fluids included.
For other fluids, you can specify the data by yourself.
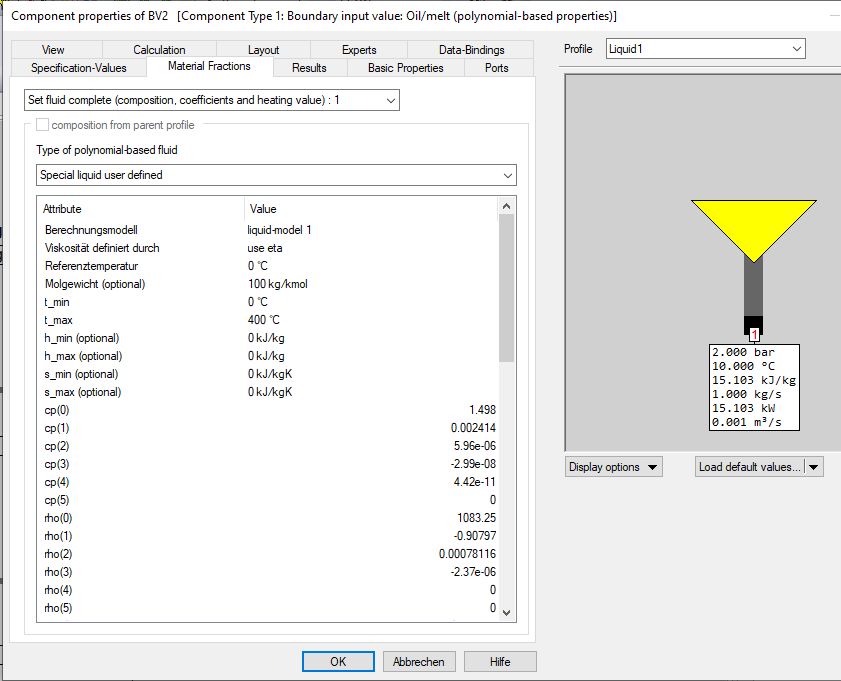
The possibility of specifying the polynomial coefficients for enthalpy and entropy is obsolete. These coefficients are no longer used.
As long as h_min and h_max (or s_min and s_max) are set to 0, this is interpreted as "no default". If they are specified, however, a solution is only searched for within the range of validity of these parameters (between h_min and h_max or s_min and s_max). In models there these limits are exceeded, error messages are output. However, these can be easily remedied by adjusting the limits accordingly.
In the case of the two calculation models "ideal gas", the enthalpy coefficient h(6) can be used for an adjustment of the enthalpy zero point.
When selecting the calculation model "liquid-model 1" (which was originally the only possible one), the following data must be specified (polynomial coefficients and range limits):
The molar weight is needed in order to be able to (depending on the fluid and calculation model)
The coefficients cppi for calculating the specific heat cp (in kJ/(kg*K)):
cp = cpp0 + cpp1*T + cpp2*T² + cpp3*T³ + cpp4*T⁴+ cpp5*T⁵
From these coefficients, EBSILON calculates the enthalpy and entropy of the fluid by integration.
The coefficients rhoi for calculating the density rho (in kg/m³):
rho = rho0 + rho1*T + rho2*T² + rho3*T³ + rho4*T⁴ + rho5*T⁵
The coefficients lami for calculating the thermal conductivity lambda (in W/mK):
lambda = lam0 + lam1*T + lam2*T² + lam3*T³ + lam4*T⁴ + lam5*T⁵
The coefficients for calculating the viscosity. The coefficients nuei for calculating the kinematic viscosity nue (in mm²/s) can be selected:
nue = exp ( (nue0 / (T+nue1)) + nue2) = e ( nue2 + (nue0 / (T+nue1)) ) (nue3, nue4 und nue 5 are unused)
or the coefficients etai for calculating the dynamic viscosity eta (in 10^-6*kg/(m*s)):
eta = eta 0 + eta1*T + eta2*T² + eta3*T³ + eta4*T⁴ + eta5*T⁵
be specified. For this purpose, the switch "Viscosity defined by" must be set to "use nue" or "use eta". The following relationship applies between the two variables
eta = rho * nue
In the case of liquid model 2, the viscosity is calculated according to this formula (with T0 = 273.15 K):
eta = exp ( (eta 0 + eta1*T + eta2*T²+ eta3*T³ + eta4*T⁴+ eta5*T⁵) * (T0/ (T+T0) ) = e ( (eta 0 + eta1*T + eta2*T²+ eta3*T³+ eta4*T⁴ + eta5*T⁵) * ( T0 / (T + T0)) )
nue = eta / rho
The coefficients psi for calculating the vapour pressure ps (in bar):
ps = ps0 + ps1*T + ps2*T²+ ps3*T³ + ps4*T⁴ + ps5*T⁵
The vapour pressure is only used for control purposes. Since the fluid should not handle a phase transition, a warning appears if the vapour pressure exceeds the pressure in the fluid.
The values of T or t and tref are each in °C.
For the other calculation models liquid-model 2, Ideal Gas and Ideal Gas with Gas Constant R, other uses of the given coefficients apply in part: (RGas= 8.31441 J/mol K):
|
|
Liquid-Model 1 | Liquid-Model 2 | Ideal Gas | Ideal Gas (Coefficients include specific gas constant R) |
|
Specific Heat cp |
cp-Polynomial(t-tref) |
cp-Polynomial(t-tref) |
cp-Polynomial (t-tref) * RGas / molar weight |
cp-Polynomial (t-tref) |
|
Enthalpy h |
h from cp-Polynomial(t-tref) |
h from cp-Polynomial(t-tref) |
( h from cp-Polynomial (t-tref) + h6) * RGas / molar weight |
h from cp-Polynomial (t-tref) + h6 |
|
Entropy s |
Sakt - Sref = |
Sakt - Sref = |
(s from cp-Polynomial(t-tref) + Pressure correction ) |
s from cp-Polynomial (t-tref) + |
|
Density Rho |
Rho-Polynomial (t-tref) |
Rho-Polynomial (t-tref) |
From molar weight |
From molar weight |
|
Thermal conductivity Lambda |
Lambda-Polynomial (t-tref) |
Lambda-Polynomial (t-tref) |
Lambda-Polynomial (t-tref) |
Lambda-Polynomial (t-tref) |
|
Vapour pressure ps |
Polynomial (t-tref) |
exp (ps0 + ps1 / (tx + ps2)); |
N/A |
N/A |